AI-Infused Document Extraction for Faster Clinical Trials
Turbocharge your clinical trial process with Conduit88. Our Azure integrations streamline document collection and analysis for life sciences, pharma, and medtech companies. Via generative AI, Conduit88 intelligently extracts critical data from client documents, enabling more accurate matching of potential participants to eligible trials.
Power BI reporting provides clear, concise data visualizations, empowering you to identify and target the right participants faster while reducing trial costs, improving enrollment, and limiting dropouts. SOC2 compliance guarantees the security of your valuable data throughout the process.
Safeguard R&D, Fortify Clinical Processes, Accelerate Delivery
Seamless integration with Microsoft Copilot and bot agents unlocks the potential for autonomous agents, further accelerating your path to trial.
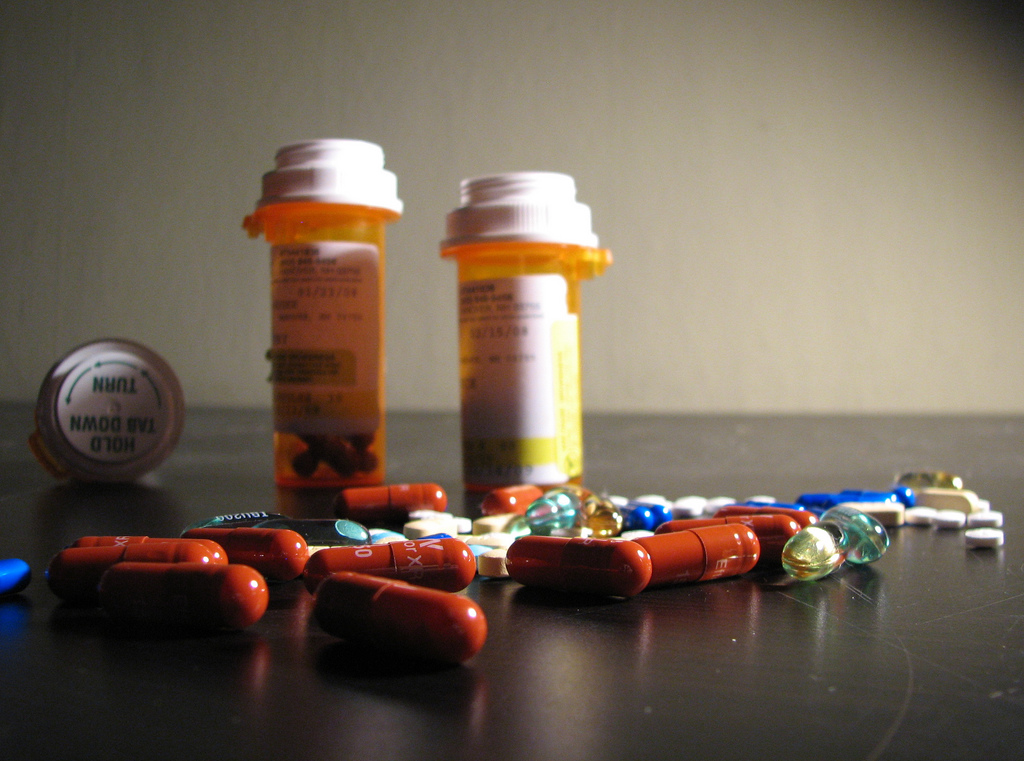
A staggering 80% of clinical trials are delayed or shut down due to enrollment challenges, with half of those delays occurring at the site level. Don’t let that be you!.
Here are a few ways life sciences and pharma companies save time with Conduit88:

Digitizing Lab and Clinical Records
- OCR converts handwritten lab notes, patient charts, and clinical trial forms into digital text.
- NCP extracts structured data (e.g., dosage, outcomes, adverse events) for analysis.
 Impact: Speeds up data entry, reduces transcription errors, and enables faster insights from clinical trials.
Impact: Speeds up data entry, reduces transcription errors, and enables faster insights from clinical trials.

Scientific Literature Mining
NLP can analyze thousands of research papers, patents, and journals to:
- Identify trends
- Extract gene-drug interactions
- Summarize findings
 Impact: Accelerates drug discovery and helps researchers stay current with emerging science.
Impact: Accelerates drug discovery and helps researchers stay current with emerging science.

Regulatory Document Processing
- Life sciences companies must comply with FDA, EMA, and other regulations.
- OCR digitizes submission documents (e.g., INDs, NDAs, SOPs).
- NLP extracts key compliance terms, flags missing sections, and ensures consistency.
 Impact: Reduces risk of regulatory delays and improves audit readiness.
Impact: Reduces risk of regulatory delays and improves audit readiness.

Patient Data Analysis
- OCR extracts data from scanned medical records, consent forms, and diagnostic reports.
- NLP identifies symptoms, diagnoses, and treatment outcomes.
 Impact: Enables real-world evidence generation and personalized medicine..
Impact: Enables real-world evidence generation and personalized medicine..

Pharmacovigilance and Adverse Event Reporting
- NLP can scan reports, emails, and call transcripts for mentions of side effects.
- Automatically classifies and routes cases for review.
 Impact: Improves safety monitoring and regulatory compliance.
Impact: Improves safety monitoring and regulatory compliance.

Clinical Trial Recruitment
- NLP analyzes patient records to match eligibility criteria.
- OCR helps digitize older records for inclusion.
 Impact: Speeds up recruitment and improves trial efficiency.
Impact: Speeds up recruitment and improves trial efficiency.

Multilingual Translation for Global Research
- NLP-powered translation tools can handle medical terminology across languages.
- Ensures consistent documentation and communication in global trials.
 Impact: Facilitates international collaboration and regulatory submissions.
Impact: Facilitates international collaboration and regulatory submissions.
Is your clinical Trial Complexity taking too long?
With a clinical trial, you need information from many sources. But if you request even a few documents, the process can drag out for months- without even mentioning potentially prickly challenges with file formats, workflows, data accuracy, and reporting.
With Conduit88, you can standardize clinical trial documentation by transforming unstructured data into the proper format via OCR and AI. That greatly simplifies patient data-sharing across Electronic Health Record (EHR) and Clinical Trial Management systems (CTMS) while also tracking every file interaction and submission for real-world data (RWD) analysis.
For regulatory compliance, multiple stakeholders must review and approve all drug development, manufacturing, inventory, and distribution documentation. However, your stakeholders may review and approve the wrong version, or do nothing at all. They may delay for weeks, then send you everything at once, and potentially blame you for taking too long.
Without sufficient management, your product-launch process can be significantly delayed. Improve your audit readiness with automated workflows with M365
Automation is key, especially when requesting approvals and signing processed documents monthly. Otherwise, the requests just pile up.
Intuitive and simple, Conduit88 provides your single source of truth. Plan, request, and approve processed documents in one place quickly and accurately.
Your team will be on the same page, knowing what has been provided or approved and what remains outstanding.
Could you give your stakeholders bulletproof mistake review and approval instructions, then free yourself from the document chase? Consduit88 gives users a gentle nudge when needed, conveniently storing the documents within your SharePoint portal.
It's easy for you,, your stakeholders, and your patients, and it won’t hurt a bit.
1500+ companies use Conduit88 to collect over 210,000 pieces of content & documents each and every month
Standard Solution
Feature Highlights

Unlimited users/ storage
Create all the client, employee, student, or recipient documents you need.
Unlimited storage costs
Remember it's SharePoint. It’s already part of your subscription.
Unlimited approvals and reminders
Set up as many Power App approvals or email reminders as you need for each document. Just don’t ignore your coworkers amidst all your newfound wizardry.

Unlimited questions & upload areas
Again- It’s SharePoint, it’s all covered. Surveys, forms, and approvals can include any number of questions or file uploads.
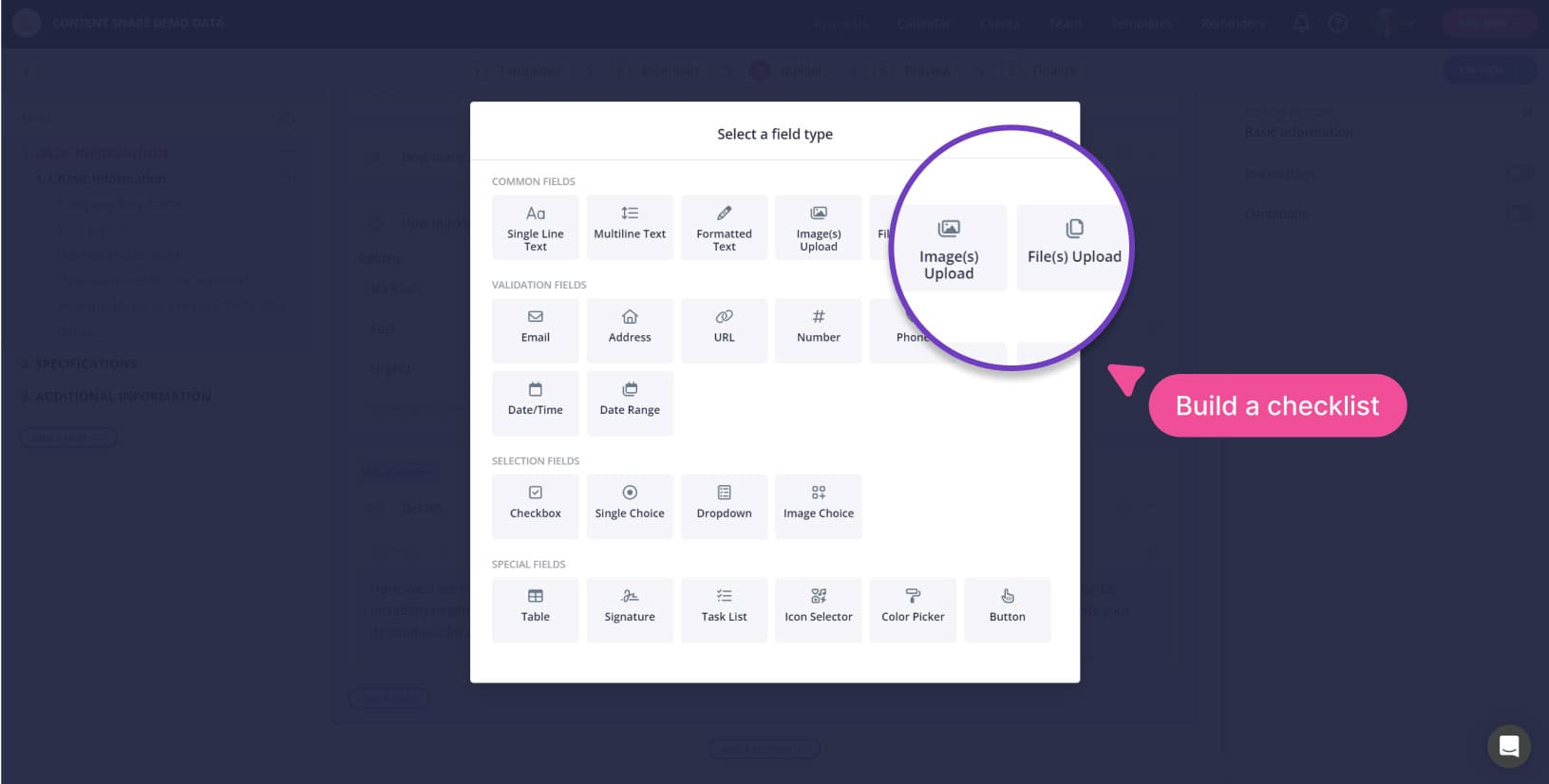
Unlimited document extraction templates
Create all the templates you want. Redact email addresses, phone numbers, or document sections as reusable templates.
Drag and drop PDF form builder
Set up as many email reminders or approvals as you need for each person to complete their work.

Simple client portal
Because it’s M365, your clients, employees, and the public get a link where they can make document requests any time, from any device.

Approval system
Approve questions or send them back for changes, if needed - all with the Power Platform that is part of your M365 subscription. Why purchase another approval tool?

Pre-made document templates
Get started quickly, without writing your own PDF forms. Use what you already have.
Integrations
Use the Power Platform for rapid workflow deployment.
- Reduced manual data entry from paper-based records
- Faster regulatory submissions, reducing time-to-market
- Improved data quality, lowering rework and compliance risks
- Efficient literature review, saving researcher time
- Enhanced patient safety, reducing liability and improving outcomes
Collect anything that’s in a document file, not just text
No matter what kind of information you put in a document, Conduit88 can help - it's not only for form field content. Use it for
- Image redaction
- Questionnaire ingestion/extraction
- Copilot inquiries
- Anything else requiring data extraction, including tables, and graphs,

Spend time on things that matter.
Let Conduit88 extract crucial document data and send automatic, periodic reminders to users.
You'll have a central place for all document content, images, and files:
- Find everything easily.
- Track changes w/o long email trails.
- Approvals are locked in to prevent unwanted changes, with the flexibility to undo and redo approvals.
- Final approvals are secured as a system of record.
Use our document extraction templates to save more time.
With just a few clicks, set up various extraction-request templates once, reusing them as needed.
Start with our template gallery, or create your own for redaction design, and extraction logic.
You can even automate the process with Microsoft M365 integrations.


Simplify document content enablement and extraction.
Say goodbye to manual PDF inputs and extractions.
Your users can annotate questions directly within each document or file. That eliminates those never-ending email trails full of questions, sporadic attachments, and often incorrect information.

 Cloud
Cloud Teams Video Conference
Teams Video Conference Murex Assessment
Murex Assessment Compliance Assessment
Compliance Assessment.png) Lucidchart to Visio Assessment
Lucidchart to Visio Assessment



